Development and manufacturing of medical devices
Giving Shape to Ideas with Japanese Manufacturing Skills
One of our aims is to strengthen domestic development and manufacturing of medical device that are innovative and safe, with our tradition of “Monodukuri” backed by strong technological capability that Japan has.
We do not rely solely on imports of medical devices from overseas, but provide one-stop solution services that support all processes from specification determination and product prototyping to manufacturing and shipping.
Meet a variety of needs
We want to switch from import-dependent procurement of medical device to domestic manufacturing that can provide stable quality.
We can provide a one-stop service from development and manufacturing to shipping judgment to the domestic market.
We want to develop products as our own branded products.
As a developer of our own products, we can create prototypes from your desired ideas.
We are looking for a domestic outsourced manufacturing.
Our manufacturing plant is equipped with clean rooms and sterilizers, and we have experience in outsourced manufacturing.
*We are licensed to MAH (Marketing Authorization Holder) and Manufacturer, so we can make various proposals to meet our customers’ needs.
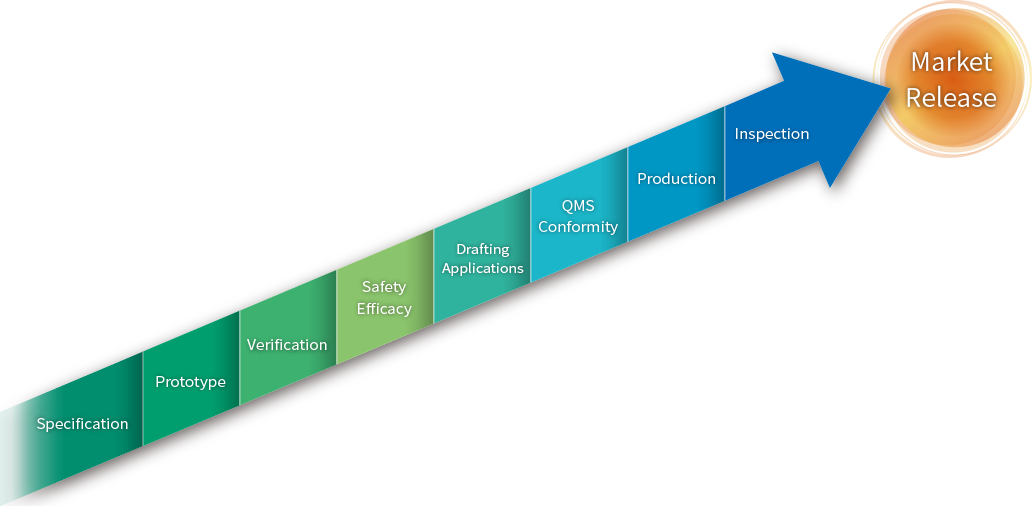
Production Process
Developmental Stage

-
Specification / Design
We will listen to customer’s requirements at meetings to determine the product specifications.
Design is done using a 3D CAD system.
-
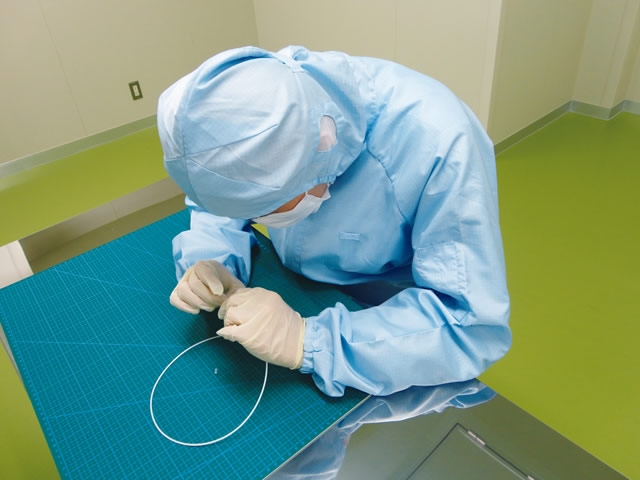
Prototype
According to our customer’s product specifications, prototyping of the product can be performed at our laboratory. For sterilized medical devices, we are also equipped with clean rooms at our Chiba Plant (Class 1,000 equivalent) and Tokyo Head Office (Class 100,000 equivalent), so we can manufacture prototypes in our clean rooms.

-
Verification
We conduct efficacy measurement for medical device. When in outsourcing a trial, we can support liaisons with various test institutions.
Manufacturing Stage

-
Production
Products are being made according to a manufacturing system based on QMS ordinance at our manufacturing site.

-
Quality Inspection
Manufactured products are meticulously inspected for quality based on the requirements of the product.
Introduction of some of our manufacturing facilities
Cleanroom

-
As for manufacturing facility especially for medical device, we are equipped with cleanroom; though medical devices are particularly sensitive to particles, this made us possible to manufacture inspect them in-house. Well-trained staff will support to improve products’ quality.
Cleanroom Specification
Class ISO8 100,000 (0.5μm) Area 52m2 Capacity 123m3 Filter HEPA filter Ventilation 29 times/h
Laser Welder

-
Laser Welder Specification
Type Nd : YAG Laser
Wavelength: 1064nmProtection Class 4
Max average output : 50wPulse Peak Power 5KW
Max Output Energy : 40JPulse Width 0.5 -20 ms
Beam divergence angle : 100πrad
Accomplishment
Jsol Electrode Catheter
 Catheter placed percutaneously and radially in the heart for temporary cardiac pacing and cardiac electrophysiology testing
Catheter placed percutaneously and radially in the heart for temporary cardiac pacing and cardiac electrophysiology testing
3Fr 20-pole deflectable type
Transesophageal Probe
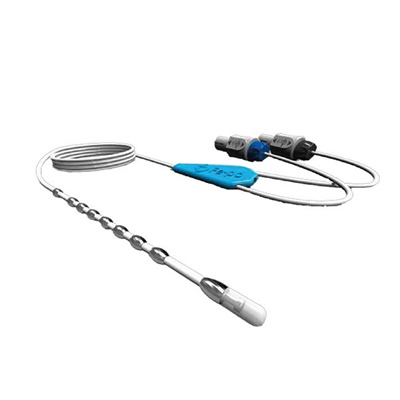 A type of catheter that enables continuous measurement of temperatures in esophagus
A type of catheter that enables continuous measurement of temperatures in esophagus
Temperature Measurement Apparatus
 A digital display device for catheter that continuously measures temperatures in esophagus
A digital display device for catheter that continuously measures temperatures in esophagus
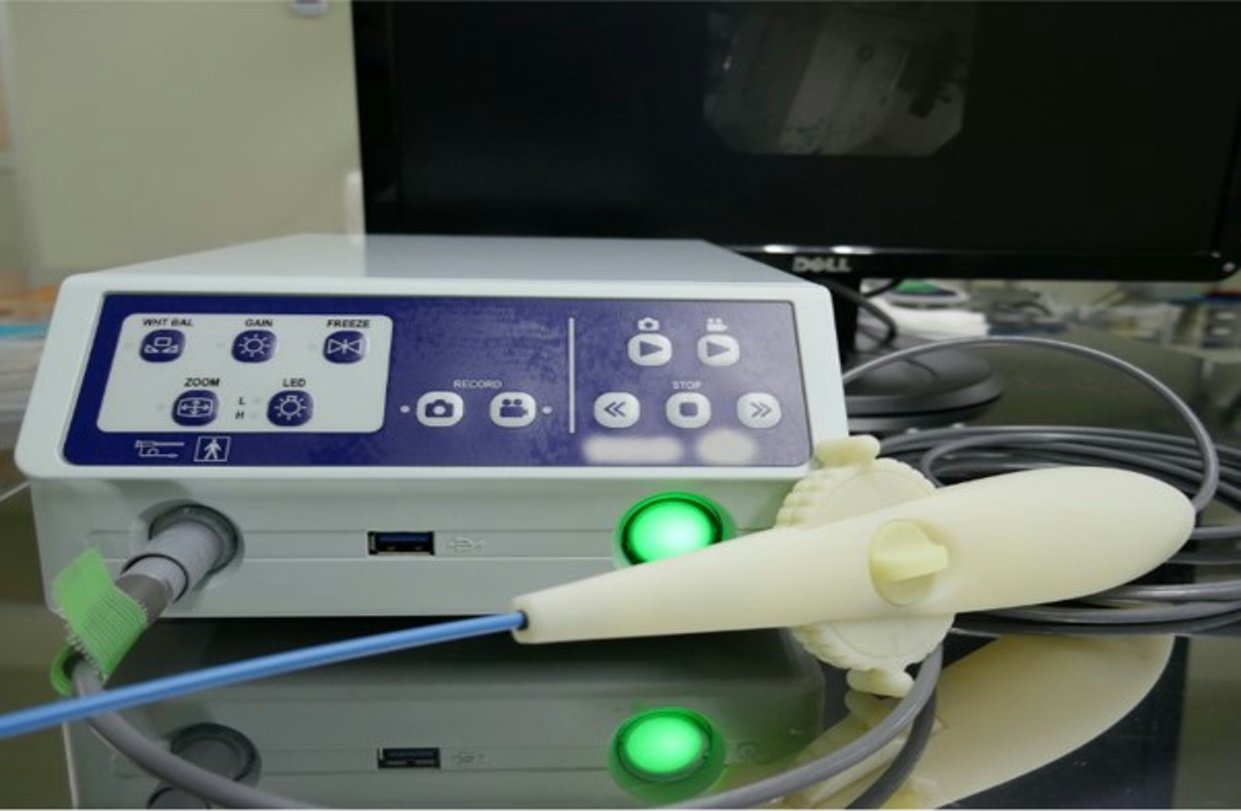
Introduction of Developed Products
We are working on the development of various products.
Here we introduce a disposable endoscope system, one of the products we are actually developing, as an example of development.