When developing a new product, we call for collaboration with small/medium size business that understand the market needs well. We can support not only the development of a new product, but also other process of medical device business –from consulting, system build-ups, manufacturing, and to market release throughout—in one place. That’s our strong point, and, we believe, it will eventually result in cost-down.
By providing our “one-stop solution” service, J-sol Medical will strive for maximizing our clients’ satisfaction. We like to support new commers to the medical device industry as well as existing companies that already import medical devices but wish to develop in-house products for lowering risks.
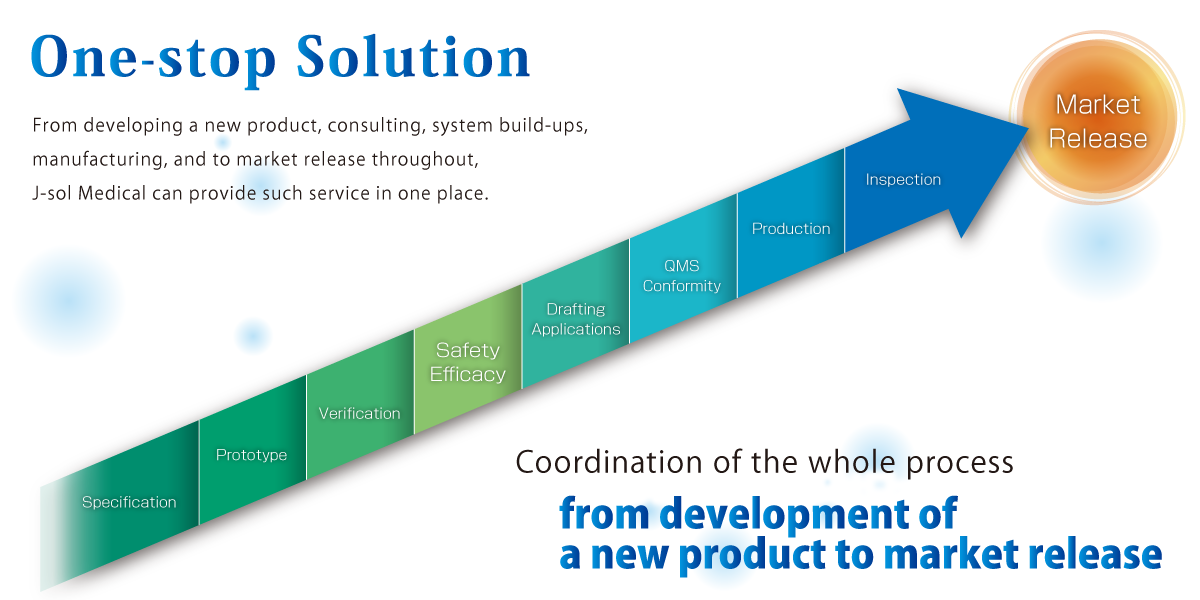
Case Example of “One-Stop Solution”
- Development Phase: Specification
- Clients’ requests hearing to decide specification of a product.
- Development Phase Prototype
- According to the product specifications, a prototype of the product can be made at our laboratory. Prototypes are manufactured in-house. We are also equipped with clean rooms at our Chiba Plant (Class 1,000 equivalent) and Tokyo Head Office (Class 100,000 equivalent), so we can manufacture prototypes in our clean rooms at the customer’s request.
- Development Phase Verification
- We conduct efficacy measurement for medical device. When in outsourcing a trial, we can support liaisons with various test institutions.
- Regulatory Assistance Services: Safety/ Efficacy
- We support liaisons with test institutions for electrical, biological and other safety testing.
- Regulatory Assistance Services: Drafting Applications
- We draft registration applications and prepare for inquiries from PMDA.
- Regulatory Assistance Service: QMS Conformity
- We support the completion of the audit by confirming the contents of SOPs of MAH (Marketing Authorization Holder) and Manufacturer, records (incoming and outgoing shipments, defects, corrective actions, etc.).
- Manufacturing
- We manufacture under a manufacturing system that complies with QMS ordinances.
- Quality Inspection
- Regularly trained staff performs detailed quality inspections based on the requirements of the product. Products that pass the inspection are packaged with regulatory labels according to QMS procedures.
- Marketing Release
- The product that has passed the quality inspection, can be distributed on the domestic Marketing after the approval of “Shipping Judgement” by MAH (Marketing Authorization Holder).





